Boron removal
in drinking water
Introduction

A boron removal plant in Cyprus
Fresh water resources are rarely containing boron, except in very delimited areas. Sea water contains 4.5 to 5.5 mg/L of boron. When sea water is desalinated to produce drinking water, the reverse smosis membranes used in this application have a very poor boron rejection.
Fortunately, boron removal with specific ion exchange resins is very effective, and several plants have been built to polish the RO permeate.
In water, boron is present as boric acid H3BO3 or sodium borate NaB(OH)4.
Plant design example
The resin
AmberliteTM PWA10 is a styrenic resin with N-methyl glucamine functionality, uniquely specific to this application. It makes a stable complex with borate:
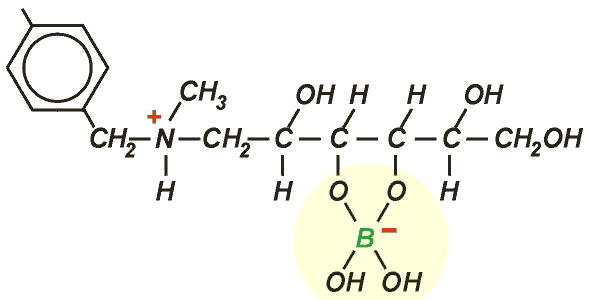
Regeneration is done with a strong acid (H2SO4 or HCl) followed by neutralisation with caustic soda.
Amberlite PWA10 is suitable for the treatment of drinking water. For industrial applications, Ambersep IRA743 is also available; it has the same functionality.
Amberlite and Ambersep are trademarks of DuPont.






